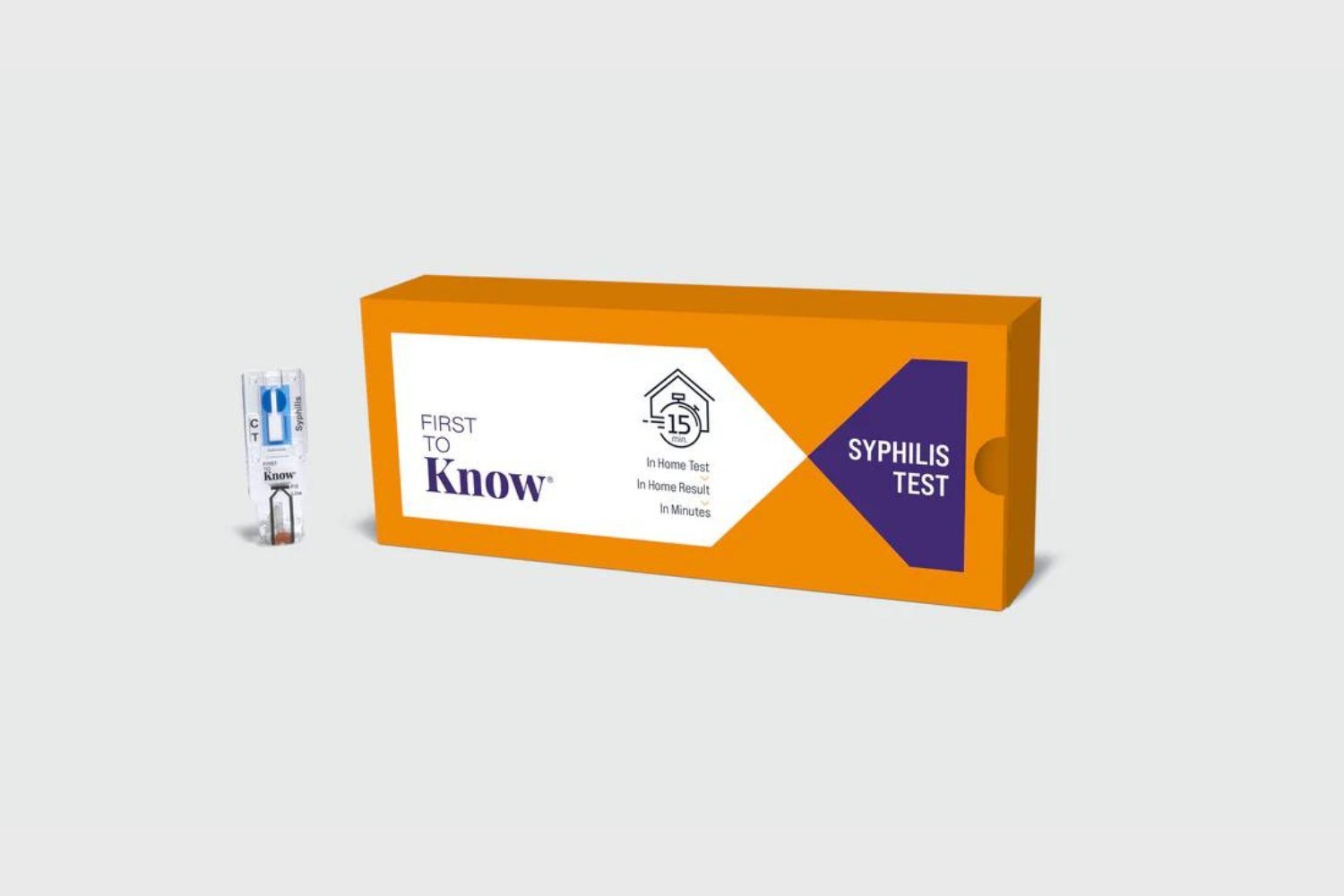
The FDA has authorized the first over-the-counter, at-home syphilis test, offering a new tool for individuals prioritizing their sexual health. This timely decision addresses the rising syphilis cases across the U.S.
The “First To Know Syphilis Test,” developed by NOWDiagnostics, marks a significant advancement. While at-home syphilis tests have existed, they typically required lab processing and physician approval. This FDA-authorized test allows individuals to independently screen for syphilis using a finger-prick blood sample, delivering results within approximately 15 minutes. In a study involving 1,207 participants, the test demonstrated high accuracy, correctly identifying over 99% of negative results and over 93% of positive results when compared to three FDA-cleared lab tests.
Increased Access and Early Detection
The FDA’s authorization emphasizes the importance of accessible testing. “Access to home tests may help increase initial screening for syphilis, including in individuals who may be reluctant to see their health care provider,” stated Michelle Tarver, acting director of the FDA’s Center for Devices and Radiological Health. Increased screening can lead to earlier diagnosis, treatment, and ultimately, a reduction in syphilis transmission.
Understanding Syphilis
Syphilis, a bacterial infection caused by Treponema pallidum, can manifest in various stages. Initial symptoms in adults may include painless sores near the infection site (genitals, rectum, or mouth). These sores typically heal, but untreated infections can progress to a secondary stage with skin rashes, swollen lymph nodes, and fever. Without treatment, the infection enters a latent phase, potentially leading to severe organ damage, including brain damage, years later. Syphilis can also be transmitted from mother to child during pregnancy, resulting in miscarriage, stillbirth, birth defects, or premature birth. Infants born with syphilis can face severe health complications or even death.
Addressing a Growing Concern
Syphilis cases have been on the rise in the U.S. along with other STIs like gonorrhea and chlamydia. While progress has been made with gonorrhea and chlamydia, syphilis, including congenital syphilis, continues to increase. In 2022, over 200,000 syphilis cases were reported, the highest number since 1950. This alarming statistic includes 3,755 cases of congenital syphilis, resulting in 231 stillbirths and 51 infant deaths.
Limitations and Recommendations
While the First To Know Syphilis Test offers a valuable screening tool, it has limitations. As an antibody test, it can detect both acute and latent infections but may also identify past, successfully treated infections. Critically, the test is solely for screening purposes. A positive result necessitates further consultation with a healthcare professional and a confirmatory lab test. The First To Know Syphilis Test is anticipated to be available in stores during the second half of 2024.