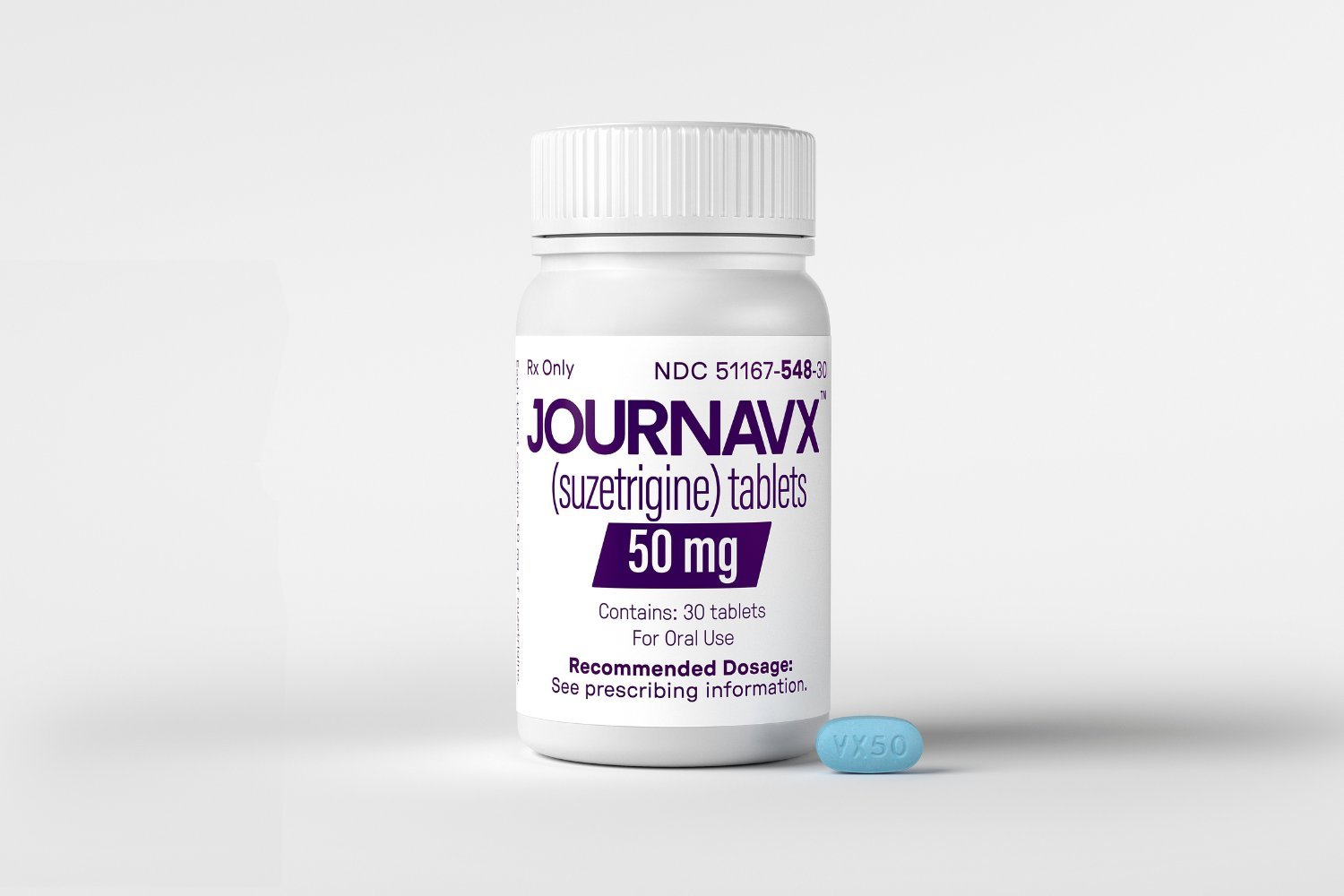
The FDA has approved a new non-opioid pain medication, suzetrigine, marketed as Journavx, offering a potential alternative to opioids for acute pain management in adults. This approval marks a significant advancement, representing the first new class of pain medication approved in over two decades and the first of its kind. This breakthrough offers hope for safer pain relief without the risk of addiction associated with opioids.
A New Era in Pain Management
The FDA’s announcement, detailed in a recent statement, highlights the importance of this new drug in addressing the opioid crisis. Jessica Oswald, a member of the Vertex Acute Pain Steering Committee, expressed optimism about Journavx’s potential to reshape pain management, offering a much-needed alternative to opioids for moderate to severe acute pain. This is particularly crucial given the devastating impact of the opioid epidemic, with hundreds of thousands of opioid overdose deaths in the U.S. since 1999, despite over 125 million opioid prescriptions dispensed in 2023 alone.
How Suzetrigine Works
Unlike opioids, which activate receptors in the brain to block pain and release endorphins (the body’s natural painkillers), leading to potential addiction, suzetrigine works differently. It selectively blocks sodium channels on pain-sensing neurons, preventing pain signals from reaching the brain without causing the opioid-like “high.” This mechanism makes it a promising alternative without the risk of addiction. Vertex Pharmaceuticals, the developer of Journavx, describes the drug as both effective and well-tolerated.
Promising Clinical Trial Results
Early clinical trials suggest that suzetrigine has no addiction potential, comparable to over-the-counter pain relievers like Tylenol or ibuprofen, according to Richard Rosenquist of the Cleveland Clinic’s Neurological Institute, as reported by NBC News. A participant in a 2023 clinical trial, Terp Vairin, reported feeling “very lucid” after taking the medication following nose surgery, without experiencing the typical opioid side effects like grogginess or nausea, as shared with Nature.
The Future of Pain Relief?
Vertex CEO and president, Reshma Kewalramani, hailed the FDA approval as a historic milestone, potentially revolutionizing acute pain management and establishing a new standard of care. However, with a price tag of $15.50 per 50-milligram pill, the accessibility and widespread adoption of suzetrigine as a true opioid alternative remain to be seen. While the medical potential is clear, the cost may pose a significant barrier to its widespread use.
The approval of Journavx provides a glimmer of hope in the ongoing battle against the opioid crisis, offering a potential pathway towards safer and more effective pain management.