Researchers have embarked on a groundbreaking clinical trial exploring the potential of a plaque-clearing drug to prevent Alzheimer’s disease in young adults genetically predisposed to the condition. This ambitious study, led by the Washington University School of Medicine, could revolutionize Alzheimer’s prevention and treatment.
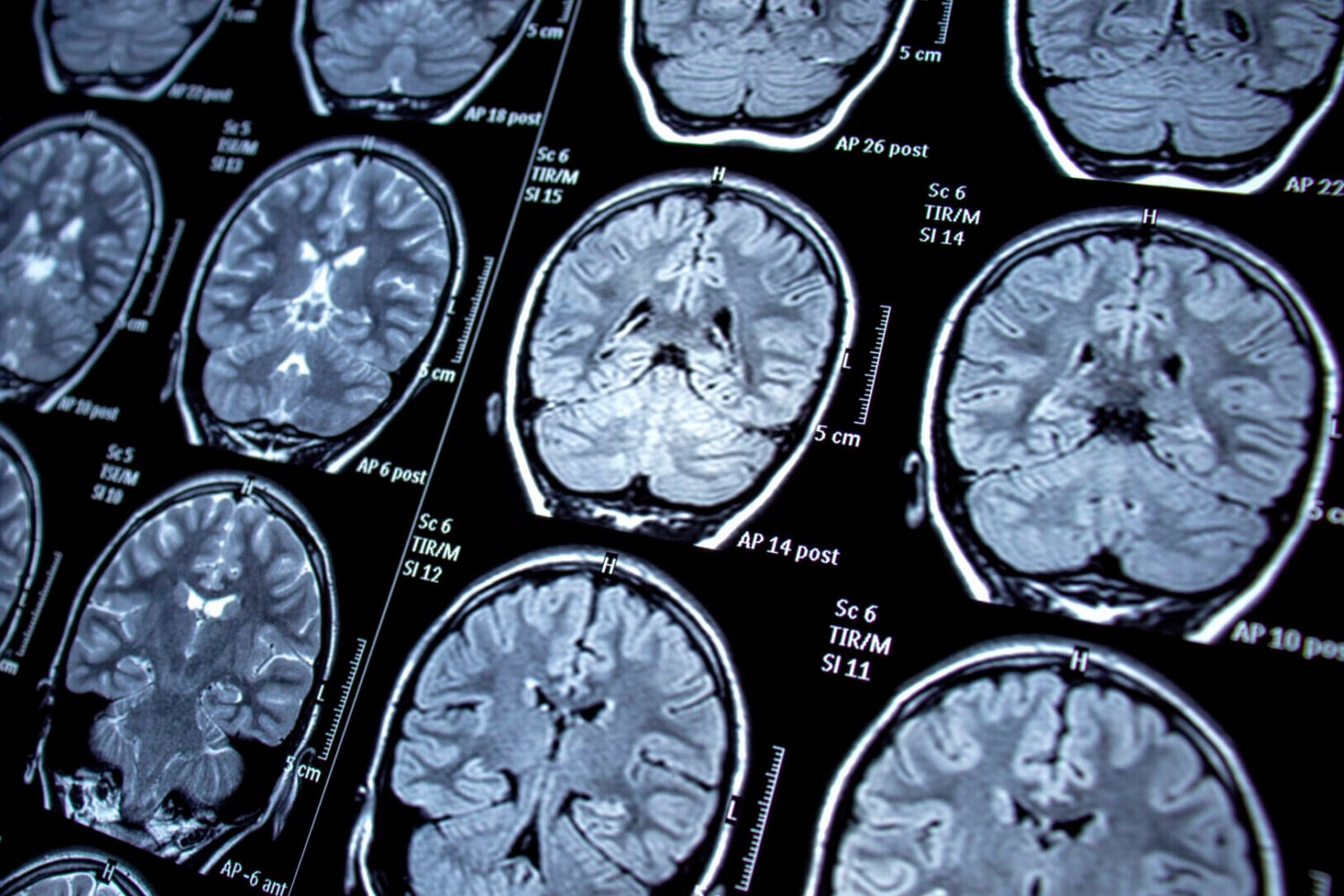
The trial focuses on remternetug, an experimental antibody developed by Eli Lilly, a successor to their recently approved Alzheimer’s drug, donanemab (Kisunla). This new drug targets misfolded amyloid beta proteins, which accumulate in the brain and form plaques, a hallmark of Alzheimer’s disease. Another misfolded protein, tau, also contributes significantly to the disease’s development.
Existing anti-amyloid treatments like donanemab offer modest benefits in slowing Alzheimer’s progression. However, amyloid plaques begin accumulating years before symptoms appear, suggesting that early intervention could be more effective. This trial directly tests this hypothesis by administering remternetug to high-risk individuals before cognitive decline manifests.
The Primary Prevention Trial involves 240 participants from families with genetic mutations known to cause early-onset Alzheimer’s. The study includes both carriers of these mutations and non-carrier relatives serving as a control group. Eligible participants are 11 to 25 years younger than the age they’re predicted to develop symptoms, typically in their 30s, 40s, or 50s.
The initial two-year phase involves randomized administration of remternetug or a placebo every three months. Following this, participants can opt to receive the drug openly for another four years. Given the participants’ young age, cognitive changes aren’t expected during the trial. Instead, researchers will monitor the drug’s impact on amyloid plaque buildup.
“My grandfather, his mother, and almost all his brothers died from Alzheimer’s,” shared 24-year-old trial participant Hannah Richardson. “I’m grateful to participate in this crucial research.”
Initial findings from the placebo-controlled phase are expected in four to five years, with the entire study concluding around 2034. Long-term monitoring of participants will continue, providing valuable insights into both early-onset and traditional Alzheimer’s.
“This innovative study has the potential to revolutionize Alzheimer’s prevention, sparing individuals and families from this devastating disease,” stated Maria C. Carrillo, chief science officer at the Alzheimer’s Association.
The researchers anticipate that this long-term study will offer critical insights into the progression of Alzheimer’s and the potential of early intervention. The results could pave the way for preventative strategies, significantly impacting the lives of those at risk.