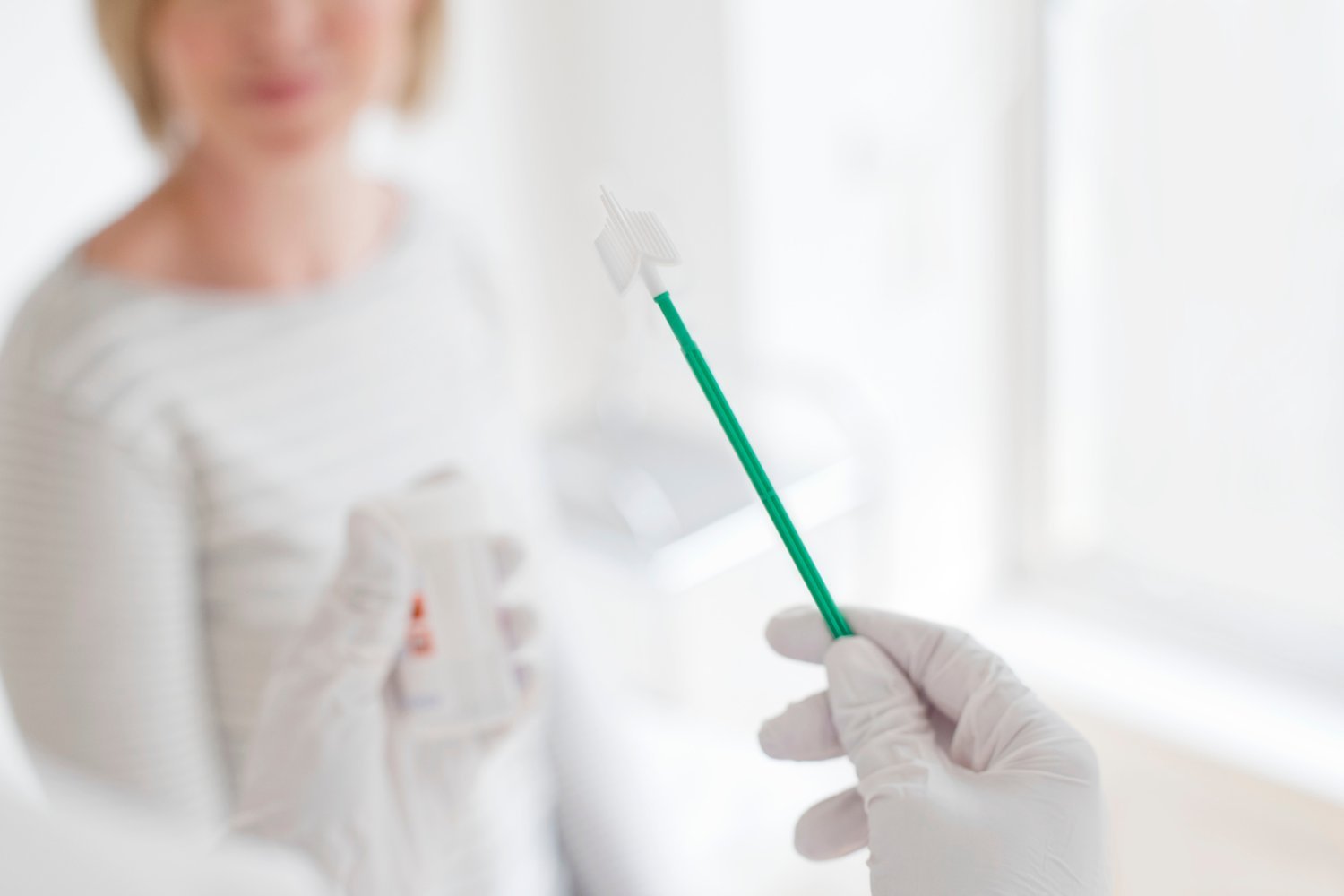
Cervical cancer is a preventable disease, yet thousands of women are diagnosed each year. Early detection through screening is crucial, but traditional Pap smears can be a barrier for many. Now, a groundbreaking at-home test offers a less invasive and more accessible alternative. The FDA recently approved the Teal Wand, developed by Teal Health, marking a significant step forward in cervical cancer prevention.
How the Teal Wand Works
Like Pap smears, the Teal Wand tests for human papillomavirus (HPV), the primary cause of cervical cancer. However, instead of a speculum exam and cervical cell scraping, the Teal Wand uses a simple vaginal swab with a sponge-like tool. This at-home collection method aims to increase screening rates by offering a more comfortable and convenient experience. Teal Health CEO Kara Egan emphasizes that this approval empowers women with a practical option that fits their lives, promoting proactive health management.
The Urgent Need for Accessible Screening
While cervical cancer deaths have decreased significantly since the mid-1970s, progress has plateaued recently. Approximately 11,500 new cases are diagnosed annually in the United States, resulting in around 4,000 deaths. Experts believe cervical cancer could be eradicated with comprehensive HPV vaccination and screening programs. However, reaching at-risk populations remains a challenge.
Addressing Barriers to Screening
Several factors contribute to the screening gap. Financial constraints, lack of insurance, and limited access to healthcare providers, particularly in rural areas where cervical cancer rates and mortality are higher, pose significant obstacles. Moreover, discomfort and anxiety associated with traditional Pap smears deter many women from seeking timely screenings. Studies show a growing preference for at-home testing, citing comfort, convenience, and flexibility as key advantages.
The Promise of At-Home Testing
Research demonstrates that at-home HPV testing is as accurate as clinical tests and effectively increases screening adherence. Studies involving mailed HPV test kits have reported substantial participation improvements compared to standard care. Teal Health’s own SELF-CERV study further supports the preference for at-home screening.
Availability and Insurance Coverage
Teal Health plans to launch its at-home testing kits next month, starting in California. The company is actively collaborating with major insurance providers to ensure affordability and accessibility. Through the Teal Health platform, individuals can request tests, receive results, and access follow-up care if needed, streamlining the entire screening process.
Conclusion
The FDA approval of the Teal Wand represents a paradigm shift in cervical cancer screening. By offering a convenient, comfortable, and accurate at-home alternative, this innovative test has the potential to close the screening gap and ultimately save lives. Increased accessibility to screening, coupled with ongoing efforts in HPV vaccination, brings us closer to the goal of eradicating cervical cancer. Teal Health’s commitment to affordability and comprehensive care further strengthens the impact of this groundbreaking development.