The landscape of birth control is overwhelmingly dominated by female options. However, Contraline, a biotech company, is making strides in developing a reversible, non-hormonal male contraceptive called ADAM. Recent news suggests this innovative approach has reached a significant milestone in its first human clinical trial.
Contraline announced promising safety and efficacy results at the 24-month mark of the trial. While peer-reviewed publication is still pending, the company plans to present further data at the American Urological Association (AUA) meeting.
How ADAM Works
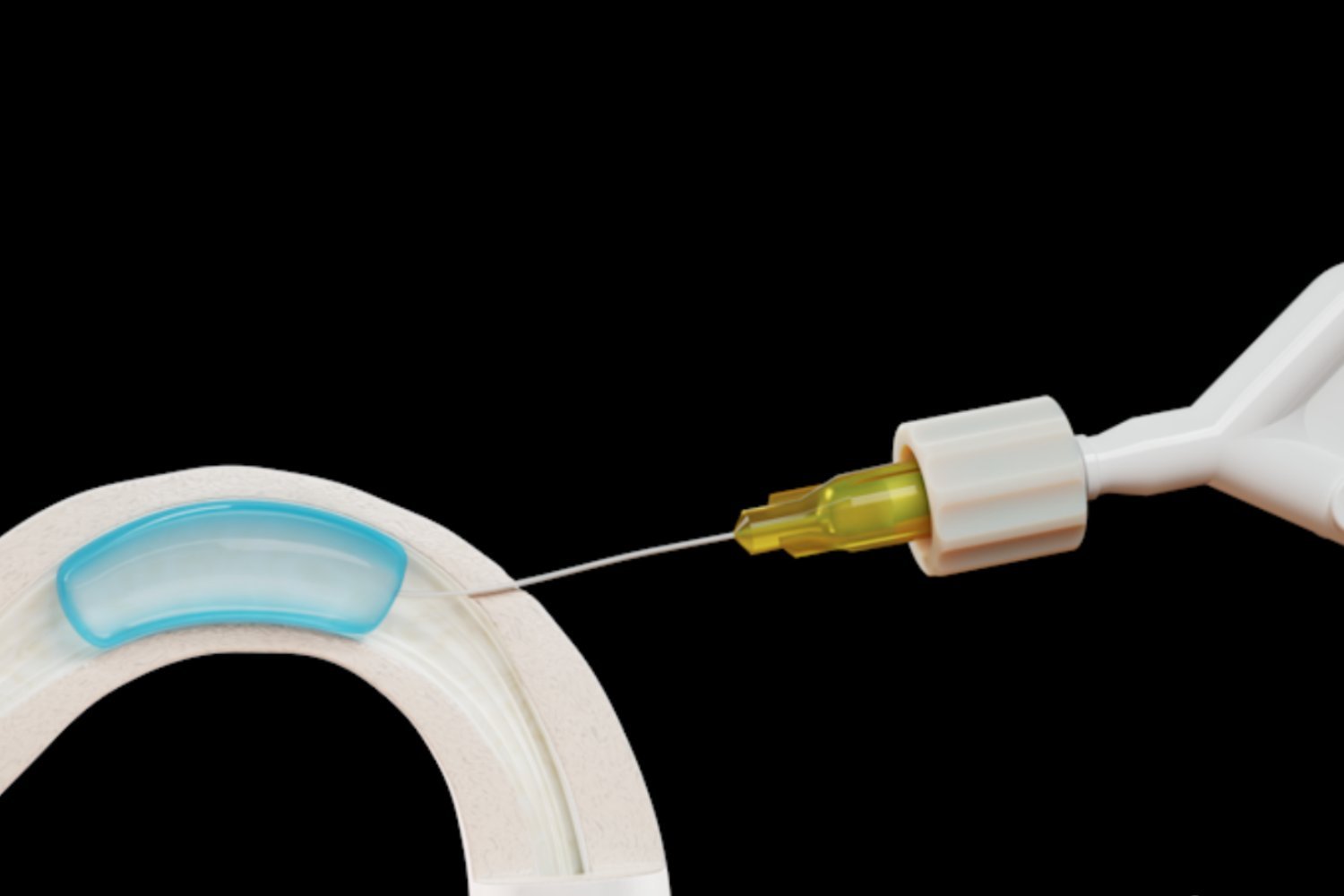
ADAM, a water-soluble hydrogel, is injected into the vasa deferentia – the tubes responsible for transporting sperm. This minimally invasive procedure blocks sperm passage without affecting ejaculation. Contraline envisions ADAM as a long-term, reversible alternative to condoms and vasectomies.
Dr. Alexander Pastuszak, Contraline’s Chief Medical Officer, emphasized the company’s goal of providing a two-year contraceptive solution for men. The 24-month findings suggest ADAM achieves this desired duration, bolstering optimism about its potential to offer men increased reproductive control.
Promising Early Results and Future Potential
The 24-month milestone highlights two trial participants who exhibited azoospermia (absence of sperm in ejaculate). Earlier data indicated a 99.8% to 100% reduction in motile sperm within 30 days of the procedure.
Kevin Eisenfrats, Contraline’s CEO, believes these results signify a potential shift in the contraceptive landscape. He envisions ADAM’s efficacy rivaling long-acting female contraceptives like IUDs, making it an appealing choice for men seeking reliable birth control.
Safety and Next Steps
So far, no serious adverse events or unexpected safety concerns have been reported. Ongoing monitoring of other trial participants at various intervals, coupled with lab and at-home sperm testing, will continue. Contraline has also received regulatory approval to initiate the second phase of the clinical trial.
Addressing Concerns and Considering Alternatives
Despite the promising results, some experts have voiced reservations. Jon Oatley, a professor at Washington State University, highlights the lack of publicly available data confirming ADAM’s reversibility and the unknown long-term effects of blocking the vasa deferentia. He also suggests a preference for oral contraceptives or patches over a procedure-based method.
Current contraceptive usage data reveals a growing trend towards long-acting reversible contraceptives (LARCs) among women. If ADAM’s safety and efficacy are definitively proven, it could potentially attract a similar demographic among men who prioritize long-term contraceptive solutions.
Conclusion
Contraline’s ADAM represents a significant advancement in male contraception. While further research and data analysis are necessary, the initial clinical trial results offer a hopeful glimpse into a future where men have more convenient and effective contraceptive choices. The ongoing trials and upcoming data releases will be crucial in determining ADAM’s potential to revolutionize reproductive health.