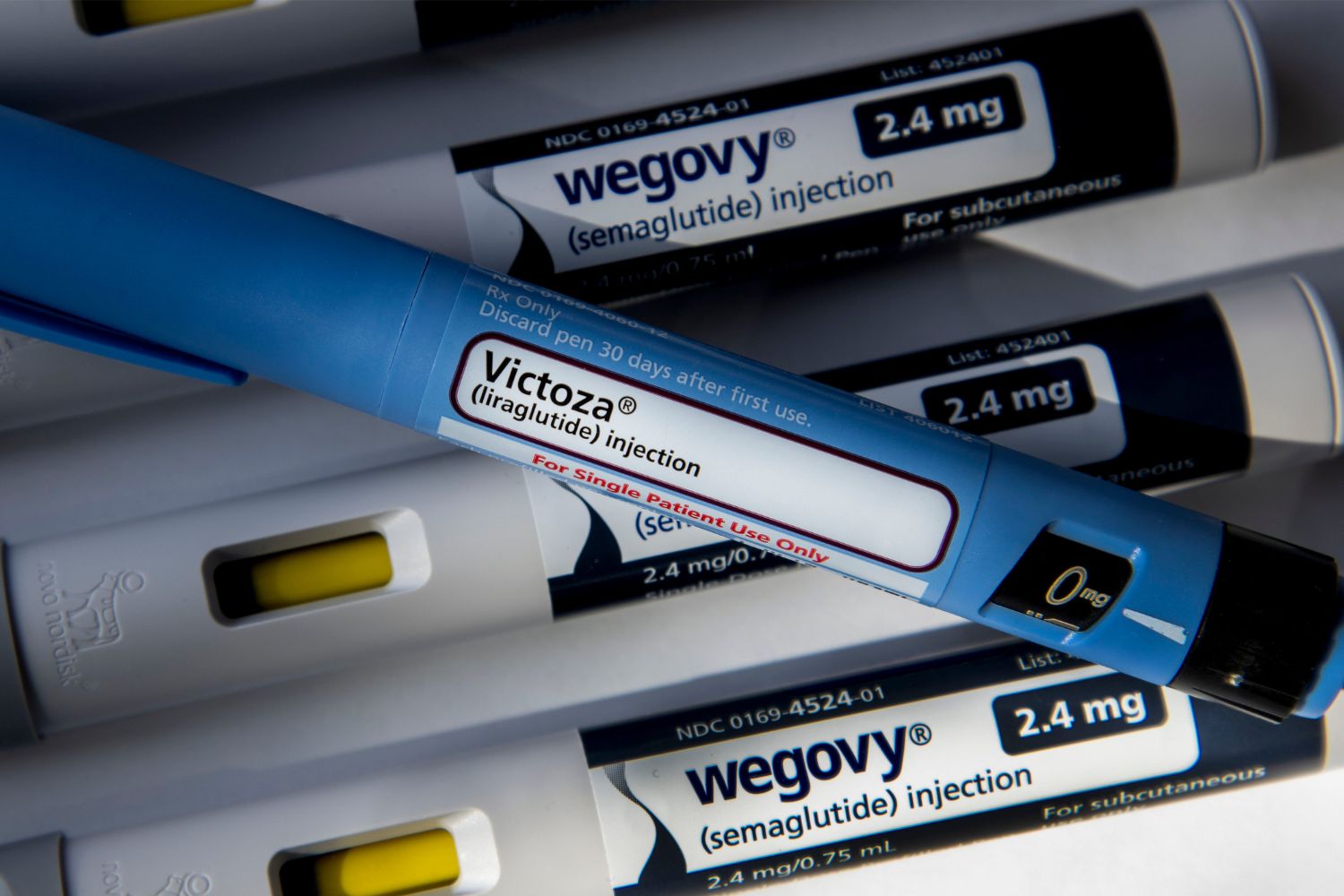
Semaglutide, the active ingredient in popular diabetes and weight loss drugs like Ozempic and Wegovy, could soon become even more convenient. New research presented at the European Association for the Study of Diabetes (EASD) annual meeting reveals a novel hydrogel formulation of semaglutide, potentially allowing for a single monthly injection instead of the current weekly regimen.
This innovative delivery system, developed by French biotech company Adocia and dubbed “Gelzepmic” (formally AdoGel® Sema), aims to improve patient adherence, a known challenge with semaglutide. While the drug has demonstrated remarkable efficacy in clinical trials, with patients losing an average of 15% of their body weight, real-world studies show only around 40% of users continue treatment for a year or longer. The weekly injections required for Ozempic and Wegovy, as well as the daily oral pill Rybelsus, may contribute to this discontinuation rate.
Adocia’s hydrogel formulation combines two biodegradable polymers to create a slow-release system. This gel, administered subcutaneously like the existing injectable versions, is designed to deliver an initial burst of semaglutide followed by a sustained release over a month.
Preclinical testing in laboratory settings and in rats has shown promising results. The gel successfully released semaglutide slowly over the intended timeframe, and importantly, the rats exhibited no signs of inflammation or toxicity, suggesting a safe and well-tolerated profile.
This breakthrough could significantly impact diabetes and obesity management. “Glucagon-like peptide-1 agonist (GLP-1) drugs have transformed type 2 diabetes care, but weekly injections can be burdensome for patients,” stated lead researcher and Adocia scientist Claire Mégret in an EASD press release. “A single shot a month could make it much easier for people living with diabetes or obesity to stick to their drug regimens, improving quality of life and reducing side effects and diabetes complications.”
The next step for Adocia is to conduct further studies in pigs, whose skin and endocrine systems closely resemble those of humans. Successful results in these trials could pave the way for human clinical trials within the next few years.
In addition to the hydrogel formulation, Adocia is also developing its own oral version of semaglutide, aiming to improve upon the bioavailability of the currently available Rybelsus. Early data suggest this new oral formulation could be more readily absorbed by the body. Both innovations hold considerable promise for simplifying and improving the effectiveness of semaglutide treatment for diabetes and weight loss.
https://www.primary-care-diabetes.com/article/S1751-9918(24)00126-8/fulltext