For the first time, scientists have observed the formation of water molecules at the smallest scale ever recorded. This groundbreaking research, conducted by engineers at Northwestern University, offers unprecedented insights into the fundamental process of water creation and holds significant implications for water generation technologies both on Earth and in space exploration.
The research team employed a novel technique involving a gas-permeable membrane and powerful electron microscopes, achieving a resolution of 0.102 nanometers, comparable to the size of the smallest molecules. This allowed them to directly visualize the birth of water molecules, a phenomenon previously unseen.
Palladium’s Role in Water Formation
The study focused on palladium, a metal known for its exceptional ability to absorb large quantities of hydrogen at room temperature and normal atmospheric pressure. While this characteristic has been recognized, the underlying mechanism remained unclear. The Northwestern team aimed to unravel this mystery.
Using their advanced imaging technique, the researchers observed hydrogen molecules interacting with palladium. Astonishingly, they witnessed the formation of tiny water bubbles on the palladium’s surface in real time.
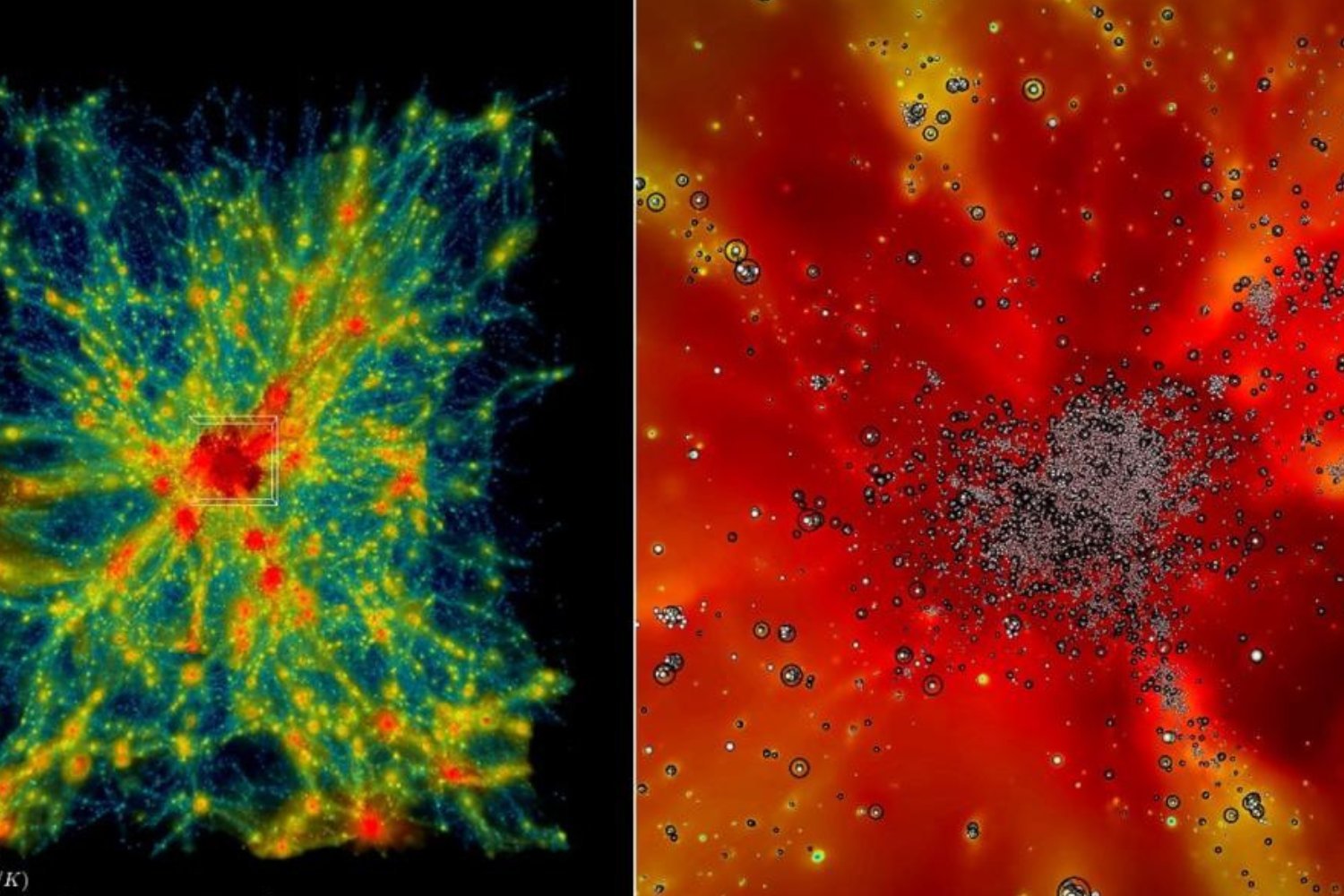
 Image of water forming on piece of palladiumTiny water bubbles forming on a piece of palladium, observed using an electron microscopy. © Northwestern University
Image of water forming on piece of palladiumTiny water bubbles forming on a piece of palladium, observed using an electron microscopy. © Northwestern University
“We think it might be the smallest bubble ever formed that has been viewed directly,” stated Yukun Liu, a PhD candidate involved in the study. “It wasn’t what we anticipated. Fortunately, we were recording, providing undeniable evidence of this remarkable event.”
Confirming Water Formation and Optimizing the Process
To confirm the formation of water, the team analyzed the energy loss from electron scattering during the process. The results matched the energy signature characteristic of oxygen bonding in water molecules, validating their observation.
The ability to visualize the process at such a fine scale allowed the engineers to fine-tune the water generation process. They discovered that pre-exposing the palladium to oxygen before introducing hydrogen slowed the reaction rate, while the opposite occurred when hydrogen was introduced first.
Implications for Future Water Generation
This discovery has far-reaching implications for water generation technologies. The optimized process, utilizing the recyclable palladium platform and abundant hydrogen gas, offers a potentially revolutionary approach to producing water in various environments, including arid regions on Earth, space stations, and even other planets.
This innovative method bypasses the need for extreme conditions like high temperatures or pressures, simplifying the water generation process. As hydrogen is the most abundant element in the universe, this method holds immense promise for future space exploration, enabling sustainable water production for crewed missions to the Moon or Mars.
Conclusion
The Northwestern University team’s groundbreaking research has provided the first direct observation of water molecule formation at the nanoscale. This achievement not only enhances our understanding of a fundamental scientific process but also opens exciting avenues for developing innovative and sustainable water generation technologies. This discovery could be instrumental in addressing water scarcity on Earth and enabling long-duration space exploration.